The only prenatal blood test that analyzes every chromosome of your developing baby and offers analysis at the level of karyotype!
The MaterniT GENOME test is the only prenatal blood test available to date that can analyze every chromosome of your baby to identify extra or missing parts of chromosomes, or other whole chromosome changes. Many of these chromosome abnormalities can severely impact the health of a baby. Through the convenience of a blood draw performed as early as 9 weeks into your pregnancy, the MaterniT GENOME test offers comprehensive information about chromosomes for your pregnancy, without the risk of miscarriage associated with an invasive procedure.
For more information regarding the MaterniT GENOME see our patient brochure here…

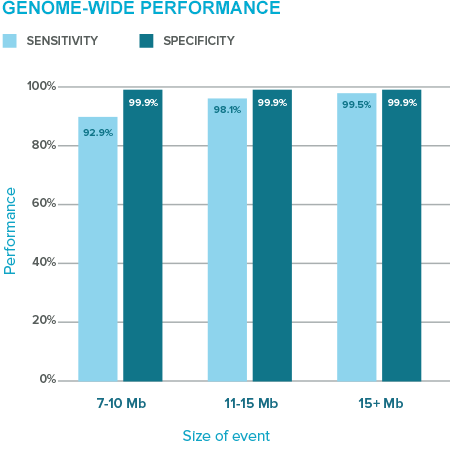
The MaterniT GENOME test starts with the ease of an ordinary blood draw, taken as early as 10 weeks’ gestation. Though not a fetal karyotype, it is currently the only NIPT that offers a level of information previously only available from a karyotype analysis. It provides information about gains or losses of chromosome material ≥ 7 Mb across the genome. The test also analyzes seven clinically significant microdeletion regions. By combining increased sequencing depth with industry-leading expertise, MaterniT GENOME offers a breadth of coverage unlike any other noninvasive prenatal test available to date.
Resolution of prenatal karyotype is often poorer than peripheral blood karyotype. Cryptic deletions or duplications larger than 7 Mb can go undetected by routine prenatal karyotype¹, the clinical consequences of which can be lethal or lead to complex, severe fetal anomalies. The MaterniT GENOME test offers a novel capability of noninvasive testing, identifying > 95% of genome-wide deletions or duplications ≥ 7 Mb. This enables the most comprehensive fetal chromosomal test currently available noninvasively.
The MaterniT GENOME test couples leading technology with unparalleled insight to offer the most scientifically advanced information available from a noninvasive test.
Sequenom Laboratories has a history of responsible innovation, with each new advancement in NIPT characterized by reliable results and supported by extensive validation studies. Validation testing of the MaterniT GENOME test builds on this history, augmenting earlier work in genome-wide analysis, to ensure highly accurate results.
The MaterniT GENOME test represents the latest scientific advancement in noninvasive prenatal testing. It can analyze every chromosome in the genome—the complete set of your baby’s chromosomes. Your health care provider may discuss the benefits of the MaterniT GENOME test with you if:
- There are concerns about chromosome abnormalities in your pregnancy
- Ultrasound abnormalities have been identified
- You have had earlier abnormal screening results for this pregnancy
- You, your partner, or a prior pregnancy or child were identified with a chromosome abnormality
- You have received inconclusive results from another fetal DNA screening test
- You have asked to learn the most information you can about your baby’s chromosomes without the risks associated with an invasive procedure
The blood is placed in a special tube containing a patented material suitable to preserve blood intact and avoid destruction of blood cells which will affect the amount of free fetal and maternal DNA in it. After careful management, the blood sample is sent to California, USA in specialized laboratories with the latest equipment for analysis.
The approach used is based on the identification and counting of large number of different DNA fragments in the plasma sample. Using a new technique called massive parallel sequencing (MPS), the exact sequence of millions of DNA fragments of the fetus and the mother is determined, and simultaneously, since the entire human sequence of our genetic material is already known, each DNA fragment that is derived, is matched with the chromosome from which it was derived.
In case of presence of a fetal aneuploidy a relative surplus or deficit of the corresponding chromosome material should be detected. Following a complex logarithmic analysis with powerful computing systems the results are issued in a simple and clear format (positive or negative) for the envisaged abnormalities.
The MaterniT GENOME test can identify common whole chromosome abnormalities like trisomy 21 (also known as Down syndrome), trisomy 18, or trisomy 13. Extra or missing pieces of chromosome material can also be found. Some of these smaller changes can be associated with rare conditions such as DiGeorge or Wolf-Hirschhorn syndromes, which often go undiagnosed at birth. Having information about such genetic conditions before birth can help ensure your baby receives the proper and necessary support.
The MaterniT GENOME test reports on many chromosome abnormalities and conditions, which include, but are not limited to:
Results are typically available 5 days after your sample has been received in the laboratory. Results are communicated clearly—as a positive or a negative result.
A positive result means that a chromosomal abnormality has been identified. Genetic counseling is recommended following any positive test result to discuss the findings and review options for further confirmatory testing. To assist in understanding an abnormal result, the MaterniT GENOME test report also has an image of the chromosome change that illustrates what was discovered.
A negative result means that no chromosome changes were identified. Though highly reassuring, it is important to note that, like many tests during your pregnancy, a negative result does not mean your baby is unaffected, as the test cannot detect all possible abnormalities.
In some instances, one of the many chromosome targets analyzed may return an “uninformative” result. This does not necessarily indicate there is a problem with your pregnancy. Most commonly, it means that the amount of fetal DNA required for that particular result is insufficient and re-testing or alternative testing may be conducted.
Some chromosome changes are associated with known conditions, while other changes may be identified in chromosome regions that are not as well defined clinically. Also, in some cases, results may indicate chromosome changes from the placenta and not the baby. Your health care provider can explain your test results and may recommend a specialized procedure, such as amniocentesis or chorionic villus sampling, to confirm either positive or negative results. You may also be referred for genetic counseling, which can help provide the context necessary to understand your test results and plan for your pregnancy.
The MaterniT GENOME test is a laboratory-developed test that was developed, validated and performed exclusively by Sequenom Laboratories. The test has not been cleared or approved by the US Food and Drug Administration (FDA). Although laboratory-developed tests to date have not been subject to US FDA regulation, certification of the laboratory is required under the Clinical Laboratory Improvement Amendments (CLIA) to ensure the quality and validity of the test. Sequenom Laboratories is certified under CLIA as qualified to perform high complexity clinical laboratory testing and is accredited by the College of American Pathologists.
No test is perfect. While the results of the MaterniT GENOME test are highly accurate, discordant results, including inaccurate fetal sex prediction, may occur due to placental, maternal, or fetal mosaicism, or other causes. Cell-free DNA (cfDNA) testing does not replace the accuracy and precision of prenatal diagnosis with CVS or amniocentesis. A patient with a positive MaterniT GENOME test result should be referred for genetic counseling and offered invasive prenatal diagnosis for confirmation of test results. A negative MaterniT GENOME test result does not ensure an unaffected pregnancy. An uninformative result may be reported, the causes of which may include, but are not limited to, insufficient sequencing coverage, sequencing noise or artifacts, amplification bias, or insufficient fetal fraction.
The MaterniT GENOME test is not intended to identify pregnancies at risk for neural tube defects or ventral wall defects. cfDNA testing for whole chromosome abnormalities (including sex chromosomes) and for subchromosomal abnormalities could lead to the potential discovery of both fetal and maternal genomic abnormalities that could have minor, or no, clinical significance. Evaluating the significance of a positive or a non-reportable test result may involve both invasive testing and additional studies on the pregnant woman. Such studies may lead to a diagnosis of whole or partial chromosomal abnormalities in the pregnant woman, which on occasion could be associated with benign or malignant neoplasms.
cfDNA testing may not accurately identify fetal triploidy, balanced rearrangements, or the precise location of subchromosomal duplications or deletions; these may be detected by prenatal diagnosis with CVS or amniocentesis.
The ability to report results may be impacted by maternal BMI, maternal weight, and/or maternal systemic lupus erythematosus (SLE). The results of this testing, including the benefits and limitations, should be discussed with a qualified health care provider.
Pregnancy management decisions, including termination of the pregnancy, should not be based on the results of this test alone.
- Di Gregorio E, et al. Large cryptic genomic rearrangements with apparently normal karyotypes detected by array-CGH. 2014;7(82).
- Zhao C, et al. Detection of fetal subchromosomal abnormalities by sequencing circulating cell-free DNA from maternal plasma. 2015 Apr;61(4):608-616.